New AI model sheds light on high-risk skin cancer: Q&A with the authors
A team led by researchers at Barts Cancer Institute (BCI), Queen Mary University of London, has developed an artificial intelligence (AI) tool that could help doctors identify which skin cancers are most likely to spread.
We spoke to Professor Jun (Alex) Wang, a group leader at BCI, and Dr Emilia Peleva, a Clinical Research Fellow and dermatologist in his team, about their new study, published in npj Precision Oncology.
What challenge were you tackling with this study?
Emilia: We are interested in cutaneous squamous cell carcinoma (cSCC), a very common form of skin cancer, which is rising in incidence as our population ages. Most cases are cured when the tumour is surgically removed, but a small proportion of tumours will return and spread (metastasise). Once that happens, treatment options are limited, and prognosis is poor.
At the time of surgery, it’s very difficult for us to know with certainty which tumours are low-risk and which will metastasise. We need better tools to identify the small number of people who will benefit from additional treatment and follow-ups.

What was your approach?
Alex: In a previous study, we looked at the changes in gene activity of these tumours and showed it could help predict risk. In this new work, we took a different angle: instead of analysing genes, we investigated whether AI could spot patterns in microscope images of tumour samples that indicate metastasis risk.
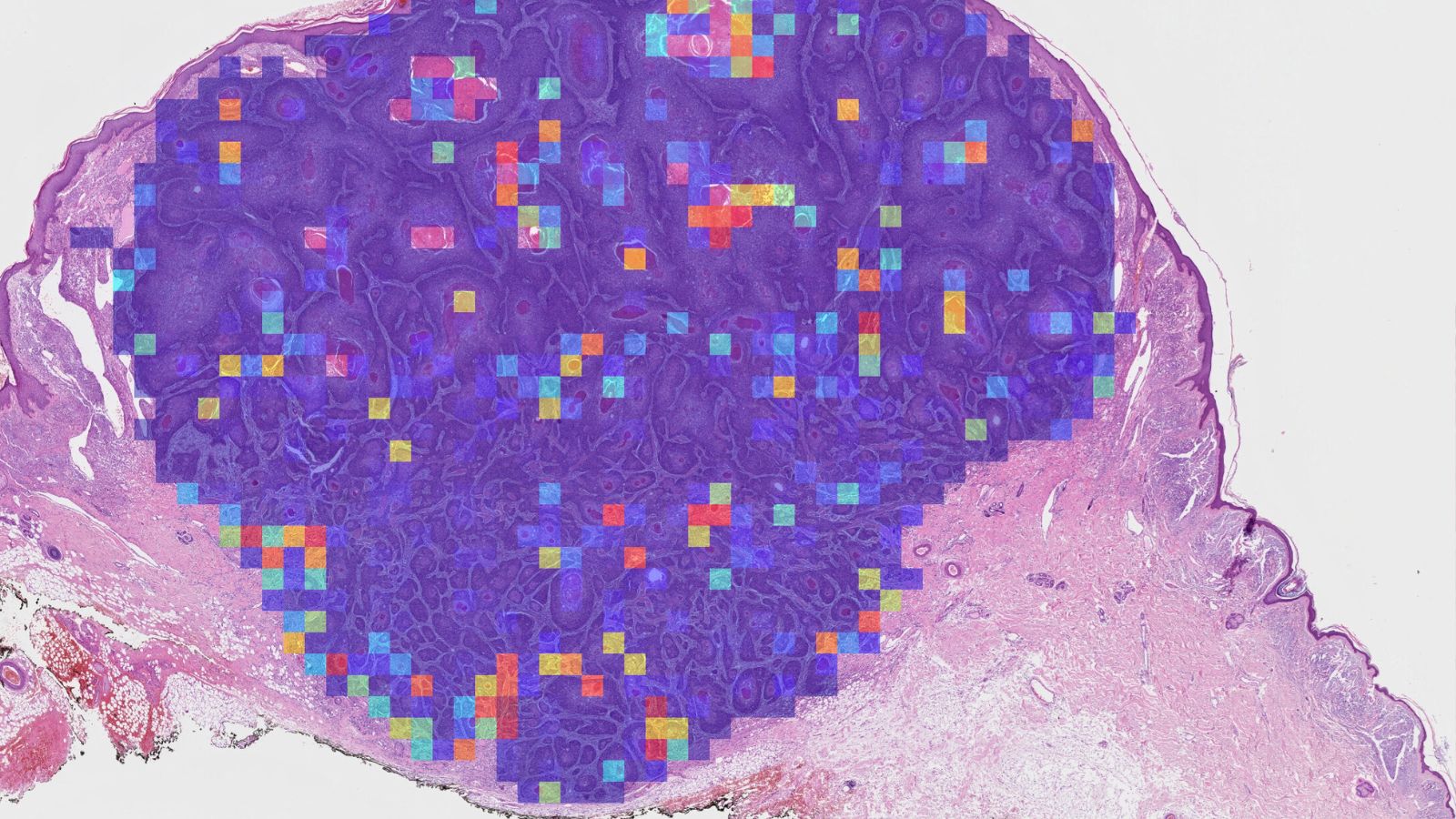
Emilia: Currently, pathologists look at patients’ tumours under the microscope and give them a grading which helps to make decisions about further treatment. But the current system doesn’t reliably pick up all the tumours which will metastasise. We investigated whether a computer model can pick up features in the digital images that the human eye can’t see, and distinguish which are linked to high-risk disease.
How did the AI tool perform?
Emilia: On a large test set of tumours it had never seen before, the AI was better at predicting which cancers would spread than the staging systems we currently use in clinics.
When we looked more closely, the AI seemed to be picking up biological features linked to how immune cells interact with the tumour. In tumours which metastasised, immune cells infiltrated more into the tumour body, rather than staying at the tumour edge. This was an unexpected finding, which may help us understand the disease at a deeper level and could open up new avenues for research.
"On a large test set of tumours it had never seen before, the AI was better at predicting which cancers would spread than the staging systems we currently use in clinics."
— Dr Emilia Peleva
What are the unique strengths of this study?
Alex: A big strength is the size and diversity of the patient group. We brought together samples and clinical follow-up data from four NHS hospitals, resulting in one of the largest cohorts of its kind. This makes the model more robust and more likely to work in real-world settings.
Emilia: Another strength is practicality. We designed it with the help of pathologists, to ensure that it works in the real world. For example, the tool works directly on standard histology slides that hospitals already prepare and, increasingly, already scan digitally. Pathologists can also see how the AI is making its decisions through heatmaps which indicate metastasis probabilities of different areas within the slide, rather than it being a total “black box.”
How could this AI tool be used in practice?
Emilia: We see this as a decision-support tool. For example, in multidisciplinary team meetings – where surgeons, oncologists, dermatologists, radiologists and pathologists review patient cases – we could add the AI score alongside all the other information to make more accurate and personalised decisions.
For low-risk patients, who have already been cured by the surgery to remove their tumours, that might mean discharging them sooner and sparing them unnecessary worry and hospital visits. For those flagged as high-risk for metastasis, it could mean closer monitoring or offering additional treatment earlier.
What are the next steps before this could reach patients?
Alex: The priority is validation in larger, international cohorts. With funding from Cancer Research UK Biomarker Project Grant, we’re now testing the model in larger groups of patients in the UK and the Netherlands. We’re also exploring whether combining the AI’s image analysis with genetic and clinical data – a multimodal approach – could improve predictions even further.
Emilia: Ultimately, our aim is to develop a tool for the NHS that helps both doctors and patients. Beyond improving outcomes for those patients at high risk of metastasis, it could help us reassure and discharge patients with low-risk tumours. By ensuring appropriate treatment for every patient, this would also optimise our use of NHS resources and ease pressure on the system as the incidence of cSCC continues to rise.
About the study
This work was led by Professor Alex Wang’s group at BCI in collaboration with Professor Catherine Harwood and Dr Hasan Rizvi at Queen Mary’s Blizard Institute and Barts Health NHS Trust. The team’s work was supported by funding from Wellcome, Barts Charity, Cancer Research UK and the Academy of Medical Sciences. For a full list of funders for all authors, please see the original paper.
Category: General News, Publications
No comments yet